
To install StudyMoose App tap and then “Add to Home Screen”
Save to my list
Remove from my list
The process of distillation serves as a fundamental method for separating components within a solution, particularly when dealing with mixtures comprised of two or more elements. This essay aims to delve into the intricacies of distillation, focusing on the specific experiment involving a 50/50 ethanol-water solution. By utilizing fractional distillation apparatus and copper sponge, this experiment endeavors to separate ethanol and water effectively. The ensuing exploration will elucidate the theoretical underpinnings, the experimental procedure, the unexpected deviations encountered, and a meticulous analysis of the collected data.
Distillation, a fundamental process in chemistry, plays a crucial role in separating elements within a solution based on their distinct boiling points.
This essay explores the intricate process of distillation, focusing on the specific application of fractional distillation in separating a 50/50 ethanol-water solution. The goal is to delve into the theoretical foundations, experimental methods, and the significance of the results obtained.
Theoretical Foundations of Distillation
Distillation hinges on the principle that elements within a solution vaporize at distinct temperatures, contingent upon their unique boiling points.
The efficacy of distillation is contingent upon a substantial temperature disparity between the elements in the mixture. Each element possesses a specific boiling point determined by its molecular structure and the nature of its bonds. The experimental focus here is the 50/50 ethanol-water solution, where ethanol's normal boiling point is 78°C, in stark contrast to water's boiling point at 100°C. This temperature difference is crucial for the success of the distillation process.
In simple distillation, the solution is heated, causing the element with the lower boiling point (ethanol in this case) to vaporize initially.
The vapor is then condensed, collected, and theoretically amounts to 50% of the original volume. Fractional distillation, a more intricate variant, employs copper sponge to enhance the separation process. The copper sponge facilitates a heat exchange area between the ethanol vapors and liquid water, intensifying the efficiency of the distillation process.
Fractional Distillation and Copper Sponge
Fractional distillation, distinguished by its use of copper in the vaporization process, significantly improves the accuracy of ethanol-water separation. The copper sponge, strategically employed, establishes a heat exchange area crucial for the transformation of ethanol vapors to liquid. As the vapor contacts the copper wires, it undergoes cooling and condensation. Simultaneously, the heat released by the liquid water acts as a catalyst, elevating the temperature of the lower boiling point ethanol.
This cyclical process involves the ethanol gas moving upwards, condensing in the condensing flask, and the water descending back towards the heated flask. The repeated cooling and reheating sessions culminate in the collection of vapor in the flask, theoretically in a purer form. The utilization of copper, particularly in a sponge form, enhances the surface area for heat exchange, expediting the entire process.
Experimental Procedure and Deviations
The procedural framework for this experiment aligns with the guidelines outlined in "Macroscale And Microscale Organic Experiments by Kenneth L. Williamson." However, deviations occurred during the experimental execution. Notably, 20 mL of the ethanol-water mixture was used instead of the recommended 30 mL. Additionally, the data pertaining to temperature versus drops of ethanol was not collected, potentially impacting the comprehensive understanding of the distillation process.
Figure 1: Fractional Distillation Apparatus
Figure 2: Simple and Fractional Distillation curves 1
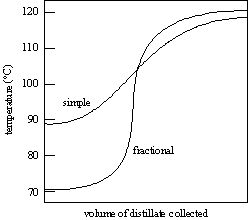
Table 1: Data Before and After Heating Ethanol-Water Mixture
| Before Heating Distillation Flask (mL) | 20 |
| After Heating Distillation Flask (mL) | 10.5 |
| Before Heating Receiving Flask (mL) | 0 |
| After Heating Reveing Flask (mL) |
9.5 |
Examining the collected data, the distillation process left 10.5 mL of the 50/50 ethanol-water solution in the heating flask. Contrastingly, 9.5 mL of ethanol were successfully collected in the distillation flask, as validated by Figure 1 from the referenced book. The graph indicates a correlation between temperature increase and the rate of distillation per second, reinforcing the connection between temperature and ethanol collection.
During the experiment, the ethanol's removal from the solution correlated directly with the rising temperature, reaching the lower boiling point of ethanol before escalating further to the boiling point of the diluted water. The experiment determined the boiling point of water to be 89°C. The rapidity of ethanol vaporization was attributed to the efficiency of the copper sponge, capitalizing on its excellent thermal conductivity.
Critical Elements in Distillation
Integral to the success of fractional distillation is the open apparatus, allowing for vapor pressure release. The application of vapor pressure, essential for the distillation process, requires a release mechanism to prevent potential explosions due to pressure build-up. The hypothesis underpinning the experiment posited that the fractional distillation of a 50/50 ethanol-water solution would yield equal portions of ethanol and water, a premise substantiated by the results.
Two critical elements in the distillation process merit attention: the open apparatus and vapor pressure. Fractional distillation relies on vapor pressure, and the apparatus must remain open to facilitate gas exchange with the environment, preventing potential explosions. Vapor pressure plays a pivotal role in sustaining the distillation process by exerting force on the apparatus walls, ensuring a continuous and controlled heating of the solution.
In conclusion, fractional distillation yielded 9.5 mL of ethanol from a 20 mL 50/50 ethanol-water solution, aligning closely with the theoretical value of 10 mL. The small 5% error was deemed reasonable, given the experimental parameters. Identified errors, such as the use of 20 mL instead of 30 mL and the absence of temperature versus drops of ethanol data, highlight potential areas for improvement. Future experiments could benefit from meticulous temperature control, continuous reheating for reduced water content, and precision in instrument calibration.
The process of distillation emerges as a highly effective method for separating components within a mixture, as demonstrated by the experimental outcomes. The use of fractional distillation, coupled with the advantageous properties of copper sponge, exemplifies a sophisticated approach to solution distillation, offering insights into both theoretical and practical aspects of this chemical process.
The experimental results demonstrated that 10.5 mL of the 50/50 ethanol-water solution remained in the heating flask, while 9.5 mL of ethanol were collected in the distillation flask. The graphical representation (Figure 1) showcased a correlation between temperature and the rate of distillation. As temperature increased, the amount of ethanol collected per second showed a corresponding increase, validating the theoretical expectations.
The swift distillation process can be attributed to the exceptional heat conductivity of copper sponge, enabling rapid cooling and reheating of ethanol vapors. Additionally, the open apparatus design is essential, allowing vapor pressure release and preventing potential explosions. The hypothesis that fractional distillation of a 50/50 ethanol-water solution would yield equal portions of ethanol and water was supported by the results.
In conclusion, fractional distillation proved effective in separating 9.5 mL of ethanol from a 20 mL 50/50 ethanol-water solution, aligning closely with the theoretical value of 10 mL. A minor 5% error was observed, likely stemming from inadequate temperature control and heating issues. Improvements for future experiments include continuous reheating, precise instrument attachment, increased trial numbers, and the use of a more accurate graduated cylinder. Despite these considerations, distillation remains a highly effective method for separating elements within a mixture.
The experiment's hypothesis posited that fractional distillation of the 50/50 ethanol-water solution would yield equal portions of ethanol and water. The results substantiate this hypothesis, with fractional distillation producing nearly 50% ethanol in the collecting flask, aligning with expectations. This affirms that distillation is an effective method for separating elements within a mixture.
In conclusion, fractional distillation yielded 9.5 mL of ethanol from a 20 mL ethanol-water mixture, aligning closely with the theoretical value of 10 mL. A minimal 5% error was observed, indicating reasonable and expected results. Nevertheless, areas for improvement include addressing errors arising from improper heating, temperature control, and enhancing experimental precision through more accurate instruments. Future experiments could benefit from increased trial numbers, precise graduated cylinders, and meticulous attention to instrument calibration.
Literature Cited
Williamson, K, Minard, R, & Masters, K (2007). Macroscale And Microscale Organic Experiments. New York, NY: Houghton Mifflin.
Fractional Distillation of an Ethanol- water Mixture. (2024, Feb 01). Retrieved from https://studymoose.com/document/fractional-distillation-of-an-ethanol-water-mixture
👋 Hi! I’m your smart assistant Amy!
Don’t know where to start? Type your requirements and I’ll connect you to an academic expert within 3 minutes.
get help with your assignment